Radiocarbon dating
Radiocarbon (Carbon-14) dating is a method of determining the age of certain archeological artifacts of a biological origin up to about 50 000 years old. The method was developed in the 1940’s. It is typically used for shells, bone, leather, fur, seeds, wood etc. There are 3 carbon isotopes that occur as part of the Earth’s natural processes; C-12, C-13 and C-14, of unstable nature; the half life of the C-14 is 5 730 years. These isotopes are present in the following amounts C12 – 98.89%, C13 – 1.11% and C14 – 0.00000000010%.
The C-14 continually formed in the upper atmosphere through the effect of cosmic ray, it is rapidly oxidised to 14CO2 and enters the earth’s plants and animals through photosynthesis and the food chain.
During the lifetime of an organism, the amount of radioactive carbon in the tissues remains stable, as the loss (through radioactive decay) is balanced by the gain (through uptake via photosynthesis or consumption ). As soon as a plant or animal dies, they cease the metabolic function of carbon uptake; there is no replenishment of radioactive carbon, only decay. By looking at the ratio of carbon-12 to carbon-14 in the sample and comparing it to the ratio in a living organism, it is possible to determine the age of a formerly living thing fairly precisely.
In addition to various pre-treatments, the sample must be burned and converted to a form suitable for the counter. The sample must be therefore destroyed in order to measure its c14 content.
When results of age determining are given, BP stands for “before present”, referring to a reference date of 1950 (date of calibration). It is presumed that the proportion of atmospheric C-14 is the same today as it was in 1950.


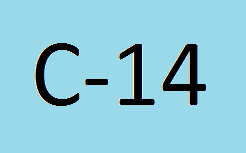
 This project (EDU-ARCTIC) has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 710240. The content of the website is the sole responsibility of the Consortium and it does not represent the opinion of the European Commission, and the Commission is not responsible for any use that might be made of information contained.
This project (EDU-ARCTIC) has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 710240. The content of the website is the sole responsibility of the Consortium and it does not represent the opinion of the European Commission, and the Commission is not responsible for any use that might be made of information contained.